Charge of io3
Iodate ion contains one iodine and three oxygen atoms.
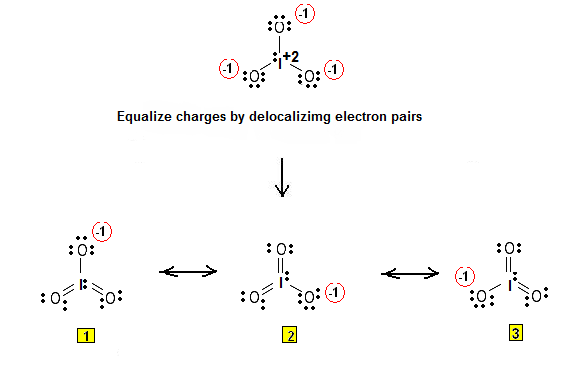
Ready to learn how to draw the lewis structure of IO3- ion? Here, I have explained 6 simple steps to draw the lewis dot structure of IO3- ion along with images. The Iodine atom I is at the center and it is surrounded by 3 Oxygen atoms O. The Iodine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge.
Charge of io3
Iodates are a class of iodine -containing chemical compounds analogous to the chlorine-containing chlorates. In these compounds, an ionic bond is formed between a metal cation and the iodate anion, which consists of one atom of iodine covalently bound to three atoms of oxygen, and carries a formal charge of Iodates are oxidizing agents, and some are used in this capacity in chemical synthesis or in personal care products such as antiseptics and deodorants. Additionally, iodates that do not contain toxic metals, such as potassium iodate or calcium iodate , can be used for iodine supplements or radioactivity prophylaxis treatments. Skip to main content. Materials by Element. Materials by Form. All Nanomaterials Quantum Dots. Materials by Application. Life Science Chemicals. About Us. Share This Page. Email Tweet Facebook. Follow Us. A Aluminum Iodate.
Email Tweet Facebook. Q: What is the chemical charge of iodate?
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate.
While the structure information of chemical compounds is critical for research and development, it is frequently difficult to find it on the web. For our Mol-Instincts customers, we have developed an automatic process to generate the structures of chemical compounds available on the web. The structure can instantly be found by our search engine below. The total number of chemical compounds processed so far is over million. We will continuously update the additional structure information of rare chemical compounds. In addition to the structure information, basic molecular information such as formula, molecular weight, and chemical identifier, e. Various options including visualization of van der Waals and exporting to a image file are available as well. Click the following link to visit an example page:. Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4.
Charge of io3
In this article we are discussing about io3- lewis structure including its drawing, hybridization, shape, pairs and some FAQS. Iodate is an oxoanion of iodine. It is formed when Iodic acid losses one proton. It has the molecular weight of Usually iodates occur in nature as salts which are generally colorless. As iodine is bigger in size and has less electronegativity than O atom I act as the central atom in this compound.
Viofo a139 pro review
That gives us a -1 formal charge for each of the Oxygens. Each electron pair : in the lewis dot structure of IO3- ion represents the single bond. Oxygen atoms have made bonds with center iodine atom. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Total electron pairs are determined by dividing the number total valence electrons by two. Valence electrons are the electrons that are present in the outermost orbit of any atom. By doing so, you will get the following lewis structure of IO3- ion. Let me explain the above image in short. Then we'll go around and fill the octets for the Oxygens. Best Answer. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Log in. From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure.
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure.
Interactive image. More answers. So it fulfills the octet rule and the iodine atom is stable. Still have questions? This is a negative ion. Best Answer. Previously Viewed. Article Talk. Here in the IO3- ion, if we compare the iodine atom I and oxygen atom O , then the iodine is less electronegative than oxygen. Opens New Window. From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure. Iodates are oxidizing agents, and some are used in this capacity in chemical synthesis or in personal care products such as antiseptics and deodorants. Also, in step 1 we have calculated the total number of valence electrons present in the IO3- ion. Remember that, there are total of thirteen electron pairs.

0 thoughts on “Charge of io3”