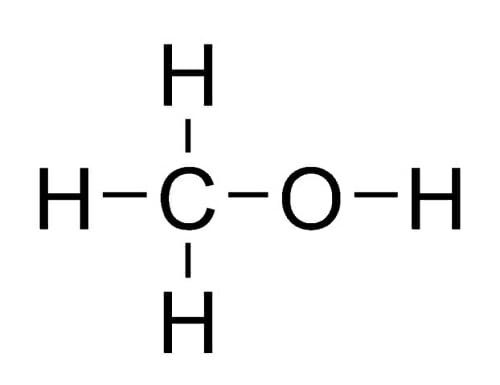
Ch3 ch2 3 ch2 oh
Wiki User. It's name is 2-amino-propanol.
An alcohol is an organic compound with a hydroxyl OH functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH , where R is an alkyl group. Alcohols are common in nature. Most people are familiar with ethyl alcohol ethanol , the active ingredient in alcoholic beverages, but this compound is only one of a family of organic compounds known as alcohols. The family also includes such familiar substances as cholesterol and the carbohydrates. Alcohols with one to four carbon atoms are frequently called by common names, in which the name of the alkyl group is followed by the word alcohol :.
Ch3 ch2 3 ch2 oh
.
Resources Leaderboard All Tags Unanswered. In these cases, the - e ending of the parent alkane is retained. Continue Learning about Chemistry.
.
An alcohol is an organic compound with a hydroxyl OH functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH , where R is an alkyl group. Alcohols are common in nature. Most people are familiar with ethyl alcohol ethanol , the active ingredient in alcoholic beverages, but this compound is only one of a family of organic compounds known as alcohols. The family also includes such familiar substances as cholesterol and the carbohydrates. Alcohols with one to four carbon atoms are frequently called by common names, in which the name of the alkyl group is followed by the word alcohol :. The carbon atoms are numbered from the end closest to the OH group. That fixes the two methyl CH 3 groups at the sixth and eighth positions. The name is 6,8-dimethyldecanol not 3,5-dimethyldecanol.
Ch3 ch2 3 ch2 oh
As noted in previously, the number of isomers increases rapidly as the number of carbon atoms increases. There are 3 pentanes, 5 hexanes, 9 heptanes, and 18 octanes. It would be difficult to assign unique individual names that we could remember. Some of the names we used earlier, such as isobutane, isopentane, and neopentane, do not follow these rules and are called common names. Atoms or groups attached to this carbon chain, called substituents , are then named, with their positions indicated by numbers.
New balance mens fresh foam x 1080 v12 running shoe
Continue Learning about Chemistry. Classification of Alcohols Some of the properties of alcohols depend on the number of carbon atoms attached to the specific carbon atom that is attached to the OH group. Two OH groups on the first and fifth carbon atoms make the compound a diol and give the name 1,5-pentanediol rule 3. Related questions. The products of propanol combustion are water and carbon dioxide. These reactions are comparatively rare. More answers. Resources Leaderboard All Tags Unanswered. If more than one OH group appears in the same molecule polyhydroxy alcohols , suffixes such as - diol and - triol are used. Alcohols are classified according to the number of carbon atoms attached to the carbon atom that is attached to the OH group.
The longest continuous chain contains five carbon atoms, so the parent hydrocarbon is pentane. The "OH" group is on "C1" , so it is 1-hydroxy.
What are the different types of organic reaction? Continue Learning about Chemistry. Related questions. Find more answers. What is the iupac name for ch3-ch2-ch2-oh? Alcohols can be grouped into three classes on this basis. All Rights Reserved. Still have questions? More answers. Alcohols are common in nature.

Many thanks for the help in this question. I did not know it.
I can not participate now in discussion - there is no free time. But I will be released - I will necessarily write that I think.
It is reserve, neither it is more, nor it is less