Ch2o lewis structure
In order to find the total valence electrons in CH2O moleculefirst of all you should know the valence electrons present in carbon atomhydrogen ch2o lewis structure as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Lewis diagrams. About About this video Transcript.
Ch2o lewis structure
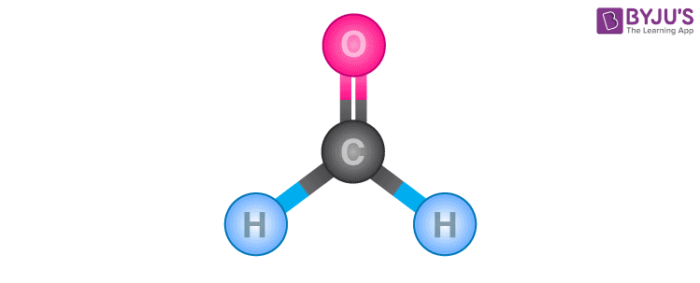
Formaldehyde is an organic compound with the chemical formula CH 2 O that appears as a colourless gas. It is the most common and simplest aldehyde, consisting of two hydrogens, one carbon and one oxygen. Lewis structure diagrams show how many valence electrons are available within an atom and participate in bond formation. It also enables visualising the behaviour of the valence electrons within the molecule and determining whether or not a lone pair of electrons exist. Determine the total number of electrons in the carbon, hydrogen, and oxygen valence shells. Hydrogen is a Group IA element with only one electron in its last shell. Oxygen is a Group VIA element with six electrons in its last shell. Carbon is a Group IVA element with four electrons in its last shell. Determine the total number of electron pairs that exist as lone pairs and bonds. Total electron pairs are calculated by dividing the total valence electron count by two. In the valence shells of the HCHO molecule, there are 6 pairs of electrons. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds. There are charges on the carbon and oxygen atoms in the centre. We will convert a single covalent bond from a single lone pair of oxygen atoms. The molecular geometry of CH 2 O is trigonal planar because the central carbon atom has no lone pair and is attached to the two hydrogen atoms and one oxygen atom through two single bonds and one double bond.
Posted 10 months ago.
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1].
CH 2 O formaldehyde has one carbon atom, two hydrogen atoms, and one oxygen atom. In the CH 2 O Lewis structure, there are two single bonds around the carbon atom, with two hydrogen atoms attached to it. And the oxygen atom with two lone pairs on it, makes a double bond with the carbon atom. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and oxygen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and oxygen has six valence electrons. Learn how to find: Carbon valence electrons , Hydrogen valence electrons , and Oxygen valence electrons.
Ch2o lewis structure
Lewis structures — also called Lewis dot formulas , Lewis dot structures , electron dot structures , or Lewis electron dot structures LEDs — are diagrams that show the bonding between atoms of a molecule , as well as the lone pairs of electrons that may exist in the molecule. The Lewis structure was named after Gilbert N. Lewis , who introduced it in his article The Atom and the Molecule. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another pairs of dots can be used instead of lines.
Fapellas
Confirm the stability of each atom. Scientists have long speculated about the how organic, or carbo. Hydrogen atoms can only form one bond, therefore, they cannot be the central atom. Why is hydrogen never the central atom? This oxygen already has these two electrons that it's sharing hanging around. Valid Lewis structures are ones where the atoms have their octets filled and their formal charges are as low as possible. So let me draw that. Your result is as below. Leave a Comment Cancel Reply Your email address will not be published. Watch Now. Why is the least electronegative atom the central atom?
Transcript: OK, this is Dr. Let's do the Lewis structure for CH2O, methanal or formaldehyde.
They also allow us to see if there are any lone pairs of electrons present. How can I know that I must draw the angle diagonally? Partition Chromatography. Total electron pairs are calculated by dividing the total valence electron count by two. And then, oxygen, it also is in the second period, and in its second shell it has one, two, three, four, five, six valence electrons. The only time we can consider hydrogen as a central atom in some capacity is for small diatomic molecules like hydrogen gas, H2, or hydrochloric acid, HCl. I will actually redraw it. Carbon is more electropositive than oxygen, so it will be the central atom. There are charges on the carbon and oxygen atoms in the center. So between carbon and oxygen, we know that oxygen is one of the most electronegative atoms, well one of the most electronegative elements on the periodic table of elements. As a result, in nature, the bond formed between carbon and oxygen in the CH2O Lewis structure is a double covalent bond. VSEPR theory considers the amount of electron groups around a central atom, both bonding and lone pairs, and determines the shape from number of those groups. The CH2O molecule has a total 12 valence electrons and all these valence electrons are used in the above sketch of CH2O.

The happiness to me has changed!
I think, that you are not right. I am assured. Write to me in PM, we will talk.