Can ionic compounds conduct electricity
Head of group Dr. Andrzej Łapiński, Prof. We are searching for new proton conductors with high conductivity and thermal stability that could be used as sources of green energy. They could be used as electrolytes in fuel cells, can ionic compounds conduct electricity, where the only by-products are water and heat.
Konkretniej - pokazuj tylko te przedmioty, dla których istnieje otwarta rejestracja taka, że możesz w jej ramach zarejestrować się na przedmiot. Dodatkowo pokazywane są również te przedmioty, na które jesteś już zarejestrowany lub składałeś prośbę o zarejestrowanie. The aim of the course is to familiarize students with the role and importance of analytical chemistry, the analytical process and its stages. Students gain an extended knowledge of the strategy, methods of sampling and preparation of samples for analysis, and analytical methods used in chemical analysis. They also learn how to check the reliability of analytical results. The aim of the course is to familiarize students with the current state of knowledge about the reactions of the various types radical, ionic, pericyclic, organometallic taking into account their stereochemical course and to show the relationship between the structure and reactivity of organic compounds. The subject Analytical chemistry acquaints students with the basics of classical methods of qualitative and quantitative analysis and allows for the practical performance of selected determinations.
Can ionic compounds conduct electricity
Random converter. Click or tap to find out! An indirect benefit of studying superconductors would be the reduction of our carbon footprint and pollution caused by burning fossil fuels, and the impact on the environment in general. Besides, using superconductors in the industry and transportation would revolutionize technology, with benefits for the entire human population. If we use superconductors, we can reduce the size while simultaneously increasing the power of all electrical devices and mechanisms such as generators, transformers, and motors. In addition, using superconductive electromagnets would help us solve the problem of thermonuclear energy synthesis. If we could do that, we would be able to make high-speed trains that travel much faster than the trains we currently have. As you can see, the interest of researchers and engineers across the globe in superconductivity is obvious. New superconductive materials are being developed. Due to their incredible conductivity recent research focuses on graphene and materials that have a similar 2-D structure. Electrical conductance is the ability of a material to conduct electricity.
Przybylska, A.
Intermolecular Comic. Skopiuj tę scenorys. Stwórz swój własny! Stwórz własną Storyboard Wypróbuj za darmo! Tekst Storyboardowy. Intermolecular Comic Stripby : cyrus david Excuse me Mrs. Of course!
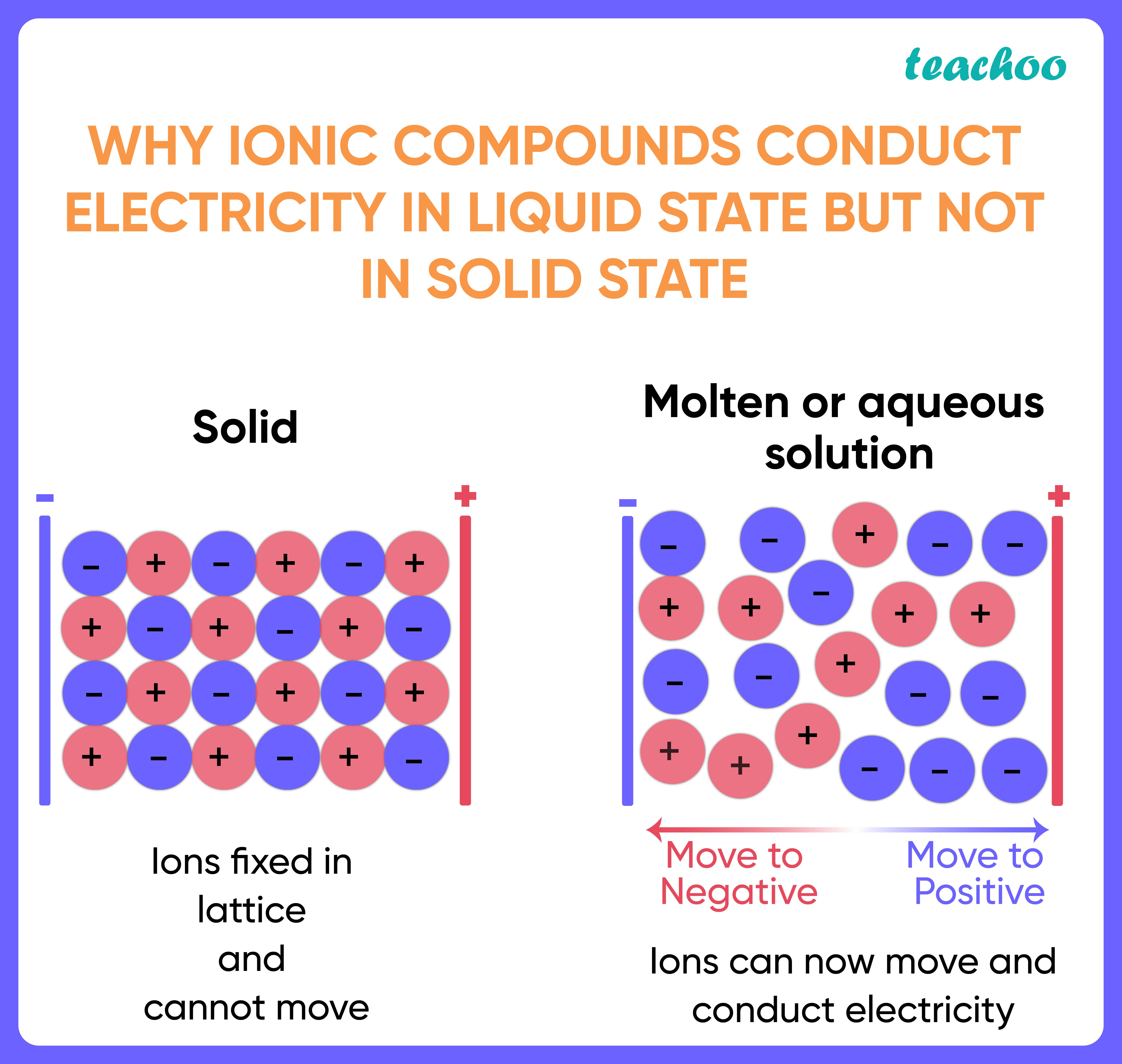
The physical properties close properties The characteristics of something. In chemistry, chemical properties include the reactions a substance can take part in. Physical properties include colour and boiling point. Listen to the full series on BBC Sounds. Ionic compounds are solids at room temperature. Melting and boiling are state close state Solid, liquid or gas. Evaporation is a change of state from liquid to gas. Energy has to be transferred to a substance in order to melt or boil it. This energy is needed to break the bonds between particles close particle A general term for a small piece of matter. For example, protons, neutrons, electrons, atoms, ions or molecules.
Can ionic compounds conduct electricity
In association with Nuffield Foundation. In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. This experiment enables students to distinguish between electrolytes and non-electrolytes, and to verify that covalent substances never conduct electricity even when liquefied, whereas ionic compounds conduct when molten. The practical works well as a class experiment, with students working in groups of two to three. There will not be time to investigate all the substances, so each group could be assigned three or four of these, and the results pooled at the end. The apparatus required for testing the conductivity of different substances when solid and molten. The covalent solids only need to be heated for a short time for melting to take place.
Loose women cast
Kozak, J. Pałys, J. If we could do that, we would be able to make high-speed trains that travel much faster than the trains we currently have. Agata Piotrowska Sc. Pyrih, A. Barszcz, T. Wydział Chemii Practical Aspects of Nanotechnology. Bursa, D. Electron structure and electron-phonon interaction in charge-transfer salts of Pd dddt 2 and Ni dddt 2. Garbacki, A.
Ionic compounds close ionic compound An ionic compound occurs when a negative ion an atom that has gained an electron joins with a positive ion an atom that has lost an electron.
Reinheimer, I. Pogorzelec-Glaser, P. Waplak, M. Zapoznaje się z poszczególnymi technikami spektroskopowymi i mikroskopowymi. Preparation and studies of transparent conductive monolayers of multiwall carbon nanotubes on quartz and flexible polymer with the use of modified Langmuir technique. Bolesław Barszcz Sc. Top Menu - EN. The process of adding these impurities intentionally is known as doping. Skibiński, D. The aim of this course is to know students with such type of materials, their structure, methods of their synthesis, their properties and their potential practical applications. Student poznaje podstawową nomenklaturę fizyczną używaną w poruszanej tematyce. Physicochemical Methods of Analysis. Chemical properties of fullerenes and carbon nanostructures. Polymer Chemistry.

0 thoughts on “Can ionic compounds conduct electricity”