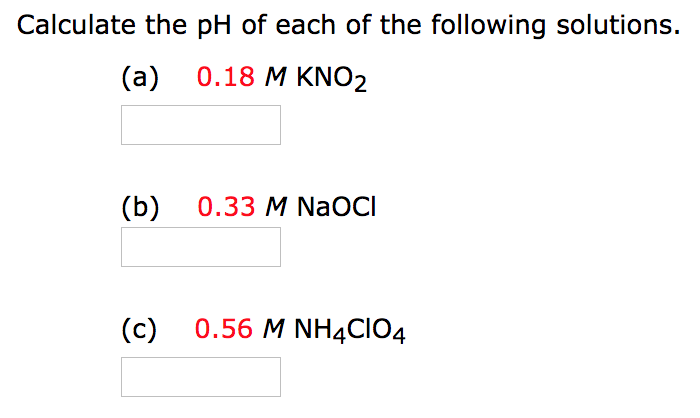
Calculate the ph of each of the following solutions
Interpretation: The pH value for each of the given solutions to be calculated. Concept introduction: The pH of a solution is defined as a figure that expresses the acidity of the alkalinity of a given solution. The value of K w is calculated by the formula. To determine: The pH value for each of the given solution of 0.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Calculate the ph of each of the following solutions
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors. Dimensional Analysis.
Gases 3h 54m.
Determine the pH of each of the following solutions. If a solution has a pH of 8. Is the solution acidic or basic? What is the molarity of hydronium ion in the solution? Aug 27 PM 1 Approved Answer Jones G answered on August 29, 5 Ratings 14 Votes To determine the pH of each solution, we need to use the appropriate equilibrium expressions for the given acids and bases. Ask your question!
With this pH calculator, you can determine the pH of a solution in a few ways. The pH value is an essential factor in chemistry, medicine, and daily life. Read the text below to find out what is the pH scale and the pH formula. In the end, we will also explain how to calculate pH with an easy step-by-step solution. Our calculator may ask you for the concentration of the solution. If you don't know, you can calculate it using our concentration calculator. You can also use the solution dilution calculator to calculate the concentration of ions in a diluted solution. A pH calculator is an invaluable educational tool, helping students and teachers alike. So, let's dive in and see how this pH calculator can simplify your life in a few simple steps. The first thing you must decide is how to calculate the pH.
Calculate the ph of each of the following solutions
The pH scale runs from 0 to 14—a value of seven is considered neutral, less than seven acidic, and greater than seven basic. To calculate it, take the log of a given hydrogen ion concentration and reverse the sign. See more information about the pH formula below. Here's a more in-depth review of how to calculate pH and what pH means with respect to hydrogen ion concentration, acids, and bases. There are several ways to define acids and bases, but pH specifically only refers to hydrogen ion concentration and is applied to aqueous water-based solutions. When water dissociates, it yields a hydrogen ion and a hydroxide. See this chemical equation below. When calculating pH, remember that [ ] refers to molarity , M.
Bannister lake conservation area
Naming Molecular Compounds. First Law of Thermodynamics -. Solubility Rules. Want to see more full solutions like this? Main Group Elements: Bonding Types. This video solution was recommended by our tutors as helpful for the problem above. Naming Alkynes. Molecular Equations. Power and Root Functions -. Amide Formation. Weak Titrate-Strong Titrant Curves. The Ideal Gas Law. Aug 27 PM. Equilibrium Constant K.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Balancing Redox Reactions: Acidic Solutions. Chemistry: An Atoms First Approach. Recent Questions in Chemistry Q:. The Quadratic Formula. The Electron Configuration Review. Nuclear Binding Energy. See Appendix 5 for Ka Problem E: Are solutions of the following salts acidic, basic, or neutral? Titrations: Weak Acid-Strong Base. The K b value is 1. Paramagnetism and Diamagnetism. Calculate the pH of a 0. Review Please. Band of Stability: Beta Decay. Arrhenius Acid.

0 thoughts on “Calculate the ph of each of the following solutions”