C2h4 sigma and pi bonds
Pages: [ 1 ] Go Down. Topic: Why does C2h4 has pi bonds but C2h6 has sigma only?
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. About About this video Transcript. Created by Sal Khan.
C2h4 sigma and pi bonds
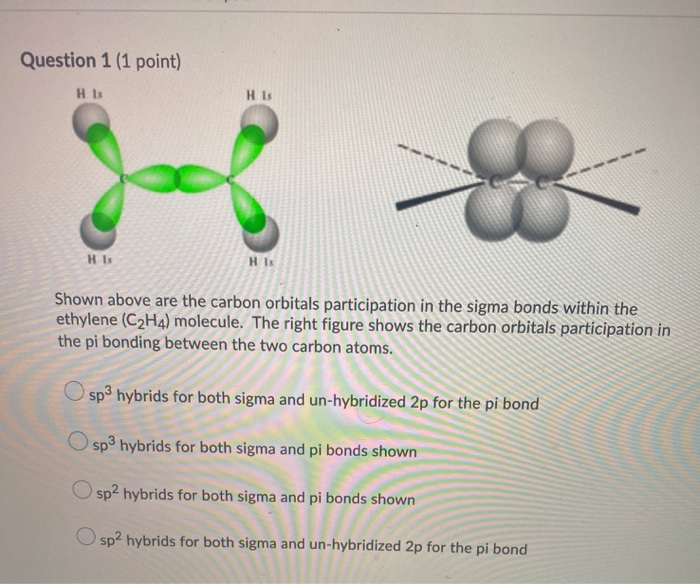
Thus far valence bond theory has been able to describe the bonding in molecules containing only single bonds. However, when molecules contain double or triple bonds the model requires more details. Ethylene commonly knows as ethene , CH 2 CH 2 , is the simplest molecule which contains a carbon carbon double bond. The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds in the ethylene molecule have been shown to be identical. Because each carbon is surrounded by three electron groups, VSEPR theory says the molecule should have a trigonal planar geometry. Although each carbon has fulfilled its tetravalent requirement, one bond appears different. Clearly, a different type of orbital overlap is involved. The sigma bonds formed in ethene is by the participation of a different kind of hybrid orbital. Three atomic orbitals on each carbon — the 2 s , 2 p x and 2 p y — combine to form three sp 2 hybrids, leaving the 2 p z orbital unhybridized. Three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the remaining electron goes into the unhybridized p z orbital. However, the unpaired electrons are contained in two different types of orbitals so it is to be expected that two different types of bonds will form. The shape of the sp 2 -hybridized orbital has be mathematically shown to to be roughly the same as that of the sp 3 -hybridized orbital.
Is it only possible only after we do experiments and find out that the C in CH4 only have single bonds so that it should be sp3, and the C in Skylander has a double c2h4 sigma and pi bonds so it is sp2? So that's this carbon right here.
When you hear the words sigma and pi bond, you might think of Greek life in college. But actually, sigma and pi bonds are types of covalent bonds. Covalent bonds happen when atoms share electrons. They are found in single, double, and triple bonds. They only exist in double and triple bonds. So, what's the difference between sigma and pi bonds?
First, we need to draw the Lewis structure of C 2 H 4. Add the remaining electrons to satisfy the octet for a more electronegative atom first. If any atoms lack an octet, make a double or triple bond to give them an octet. The two carbon atoms must be connected because hydrogen cannot have more than one bond and therefore, it cannot be between the two carbon atoms. So, there are 2 left which we put on the carbon atoms:. Now, what you need to remember is that species with unpaired electrons are called radicals and these are very unstable, and therefore, these electrons are used to make a new bond between the carbon atoms:. There is a double bond between the carbon atoms.
C2h4 sigma and pi bonds
The hybridization model can explain covalent bond formation in a molecule. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds. The two bonds differ in the way in which overlapping occurs. Various bond properties like bond length, bond energy, and bond enthalpy depend on how orbitals overlap. The electron density is concentrated between the nuclei of the bonding atoms. Sigma bond is the strongest covalent bond, owing to the direct overlapping of the contributing orbitals. The bonding electrons are usually referred to as sigma electrons. Generally, all single bonds are sigma bonds.
Shaking gif
Periodic Trends. Briana Simms. I'll draw it a little bigger so it's kind of pointing out at us, right? Buffer Solutions. With this information, you can easily count sigma and pi bonds. Thin-Layer Chromatography. The unhybridized 2 p z orbital is perpendicular to the plane of the trigonal planar sp 2 hybrid orbtals. Sponsored Links. Organic Synthesis. That's what it causes. It just has one electron in its 1s orbital. The sigma bonds formed in ethene is by the participation of a different kind of hybrid orbital. That's the best I could think about it. Ideal and Real Gases. PS I clearly understand I should've been giving a better introduction but I literally have 2 months and to cover 2 years worth of studying in it for my GCSE exams im giving accelerated Now coming to the actual question: Here is what I know: S and P orbitals are hybridized to form new orbitals when with c2h4 and c2h6 because carbon only has two unpaired electrons.
This page explains how double covalent bonds arise.
We're still forming four bonds. The main differences between sigma and pi bonds has to do with their formation and strength. Each of the four carbon-hydrogen bond in ethylene are equivalent has have a length of Reactions of Aldehydes and Ketones. Catalysts Organic Chemistry. Privacy Policy Terms and Conditions. To kind of get a better visualization of how that might work, let's think about ethene. When covalently bonding, WHY do not all the orbitals hybridize? So you have-- let me do it a different color. Now let's just wind the clock back a bit, we had four sp3 orbitals in an c2h6 , three of which already have bonded and there's one sp3 orbital left from where the carbon-hydrogen bond was broken because of oxidation of C2h6. The two atoms share one pair of electrons in a sigma bond. Although each carbon has fulfilled its tetravalent requirement, one bond appears different. Oh, maybe this purple color. In a pi bond, since the orbitals overlap in two areas you can't rotate the atoms without breaking the overlap and thereby breaking the bond.

You are not right. I am assured. I can defend the position. Write to me in PM, we will communicate.
This variant does not approach me.
Here there can not be a mistake?