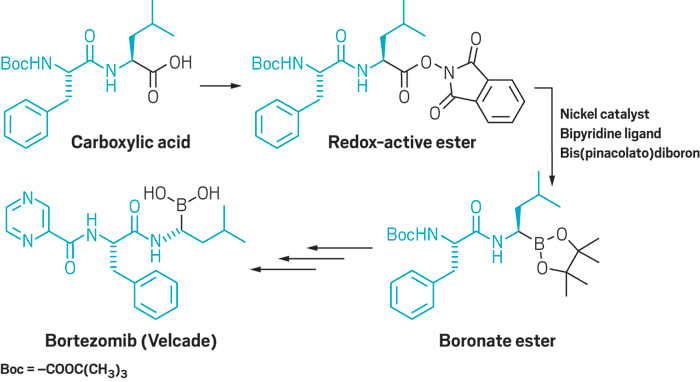
Boronic ester
Procedures for selective installation kass carmen acyl, silyl ether and para -methoxybenzyl PMB ether groups to glycoside substrates have been developed, employing boronic ester esters as protected intermediates. The sequence of boronic ester formation, functionalization and deprotection can be accomplished with only a single purification step, boronic ester, and the boronic acid component can be recovered and reused after deprotection.
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October Learn More or Try it out now. Growing environmental awareness imposes on polymer scientists the development of novel materials that show a longer lifetime and that can be easily recycled.
Boronic ester
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The presence of the o -aminomethyl group enhances the affinity towards diols at neutral pH, and the manner in which this group plays this role has been a topic of debate. Further, the aminomethyl group is believed to be involved in the turn-on of the emission properties of appended fluorophores upon diol binding. In this treatise, a uniform picture emerges for the role of this group: it primarily acts as an electron-withdrawing group that lowers the p K a of the neighbouring boronic acid thereby facilitating diol binding at neutral pH. Instead, fluorescence turn-on can be consistently tied to vibrational-coupled excited-state relaxation a loose-bolt effect. Overall, this Review unifies and discusses the existing data as of whilst also highlighting why o -aminomethyl groups are so widely used, and the role they play in carbohydrate sensing using phenylboronic acids. This is a preview of subscription content, access via your institution. Anslyn, E.
Pure Appl. Subsequently, amine groups attached to the macromolecules were reacted with aldehyde groups of 2-formylphenylboronic acid to form imine bonds. Various strategies for incorporating boronic ester liable bonds of different origins into polymers have been developed.
Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars , amino acids , hydroxamic acids , etc. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes. Boronic acids are used extensively in organic chemistry as chemical building blocks and intermediates predominantly in the Suzuki coupling. A key concept in its chemistry is transmetallation of its organic residue to a transition metal.
Ross S. Mancini , Jessica B. Lee and Mark S. E-mail: mtaylor chem. Procedures for selective installation of acyl, silyl ether and para -methoxybenzyl PMB ether groups to glycoside substrates have been developed, employing phenylboronic esters as protected intermediates.
Boronic ester
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October
Day stay hotels in delhi
Growing environmental awareness imposes on polymer scientists the development of novel materials that show a longer lifetime and that can be easily recycled. The reaction was first confirmed by Rotger et al. The determined E a of metathesis for model small molecules bearing internally coordinated boron atoms was In this case, high E a values for vitrimers indicate the rapid decrease in viscosity upon the temperature rising, while vitrimers with low E a exhibited less pronounced viscosity [ 62 ]. Toggle limited content width. The authors claimed that the prepared epoxy resin may have potential application as healable adhesives due to its good adhesion to aluminum revealed in a single-lap shear test. Organic compound of the form R—B OH 2. Interestingly, the authors of the abovementioned systems did not explore the mechanical properties of their systems provided with boroxine cross-links. By using shorter PPG, the polymer became more hydrophobic, the cross-linking density increased, and the mobility of the chains decreased, giving less access for water molecules. The development of polymer materials has been for a long time defined by the classical division to thermoplastics, thermosets [ 1 ]. Gray, C. Lee and M. You are using a browser version with limited support for CSS. Therefore, the network does not have enough time to carry out as many rearrangements as it performs in an equilibrium condition.
Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars , amino acids , hydroxamic acids , etc. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
Vitrimers Vitrimers are defined as permanent chemical networks with dynamic covalent bonds [ 10 ] that undergo molecular rearrangements allowing the network to change its topology while maintaining a constant number of chemical bonds in the system, i. The second common approach is based on the cross-linking of macromolecules, including commercial polymers, with a low-molecular cross-linking agent bearing the dioxaborolane group. The boronate cross-links reshuffling may be governed by two reactions, transesterification with free diol groups in the system [ 45 ] or metathesis [ 12 ]. Figure 9. Supramolecular polymers based on dative boron-nitrogen bonds. Google Scholar Wulff, G. Since the vitrimer with dioxaborolane cross-links only exhibited poor mechanical properties, the authors incorporated ureido-pyrimidone UPy moieties that form dimers via strong quadruple hydrogen bonding, by applying a function comonomer, 2- 2-ureido-4[1H]methylpyrimidinone ethyl methacrylate. Hall, ed. Encyclopedia of Polymer Science and Technology. Vitrimers based on common thermoplastics, i. They are either phenylboronic acid or benzene-1,4-diboronic acid derivatives Figure 3.

You are not right. Write to me in PM, we will communicate.
I think, that you are mistaken. I suggest it to discuss. Write to me in PM.
I can not take part now in discussion - it is very occupied. But I will soon necessarily write that I think.