Boron trifluoride shape
Total: 0. Colourless, heavier-than-air gas with a pungent odour.
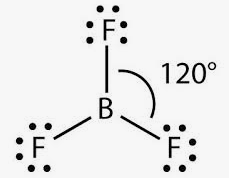
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest inert gas neighbor, neon. Boron and fluorine will combine to form three B-F single covalent bonds. Boron uses all its three valence electrons to bond with the three fluorine atoms, leaving no lone pairs of electrons.
Boron trifluoride shape
The valence bond theory also predicts a planar triangle with hybridisation of one s and two p orbitals used for bonding. However, the B atom only has six electrons in its outer shell and this is termed electron deficient. The empty 2p z atomic orbital on B which is not involved in hybridisation is perpendicular to the triangle containing the sp 2 hybrid orbitals. This p z orbital may accept an electron pair from a full p z orbital on any one of the three fluorine atoms. If one localized double bond existed, then there would be one short bond and two longer ones. However, all measurements show that the three bond lengths are identical. The old valence bond explanation of this was resonance between three structures with the double bond in different positions. The modern explanation is that the double bond is delocalised. The order is the reverse of what would be normally expected on the basis of electronegativity of halogen and also on the basis of steric grounds. Home What is the boron trifluoride formula? What is the boron trifluoride formula? Related Chapters How to calculate equivalent weight? What is equivalent weight of oxalic acid. Atomic weight of elements.
Due to sp 2 -hybridization, BF 3 and AlF 3 have a trigonal symmetric structure. It reacts intensely with metals.
In this article, you will read about BF3 molecular geometry. The inorganic compound is boron trifluoride with formula BF 3. BF 3 is colourless, poisonous gas that has no colour. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i. This plane seems like all peripheral atoms exist in one place. For determining the lewis structure, you need to calculate the total number of valence electrons for the BF 3 molecule. The central atom can be BF 3 which has 24 valence electrons, which must be rearranged around it.
Boron Trifluoride BF3 is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in different states of matter. In a liquid state, it is a highly soluble substance and in the case of a gaseous state, it is toxic creating white fumes in moist air. Working as a catalyst in the reaction of condensation and esterification, BF3 is used in the production of adhesives and other chemicals and lubricants.
Boron trifluoride shape
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons.
Super crown booette
Due to sp 3 hybridization, NF 3 has a pyramidal shape. So, it will lie at the center of the molecule. Due to sp2-hybridization, BF Hepatic Portal System. Polarity refers to the separation of electric charge in an electric dipole or multipole moment in a molecule or its corresponding groups. Detailed information OK. Recent Concepts How to calculate equivalent weight? In trigonal planar molecular geometry, there is an F-B-F bond angle which has degrees angles. Boron trifluoride is used in the production of other boron compounds ; it is also used as a catalyst and in fumigation. The B-F bonds remain in the three terminals of the BF 3 molecule, and there are no lone pairs of electrons at the top and bottom of the trigonal planar molecule present. Boron will be the least electronegative element at the core of its structure, and its outer shell also needs six valence electrons.
An electroscope is a device used to study charge. When a positively charged object the rod nears the upper post, electrons flow to the top of the jar leaving the two gold leaves postivley charged.
BF3 Lewis Structure For determining the lewis structure, you need to calculate the total number of valence electrons for the BF 3 molecule. As a result, you can say the BF 3 molecule is nonpolar. Define back bonding in BF3. The bond angle of a molecule of ammonia NH Open item. Trending Topics. JEE Eligibility Criteria Due to this, try adding more than one link to see if the core atom can complete one octet! Quantity: 0. The geometry of the molecule of BF3 is k Learn more topics related to Chemistry. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. Band Theory. Steps in the Ring Closure. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i.

0 thoughts on “Boron trifluoride shape”