Bond angle of bh3
Wiki User.
Trending now This is a popular solution! General, Organic, and Biological Chemistry 3rd Edition. Skip to main content. Homework help starts here! Publisher: Cengage Learning. What is HCH bond angle implied by this Problem 17CTQ: Indicate the bond angle and shape about each central atom.
Bond angle of bh3
In this video, you will learn about the different types of molecular geometry and their ideal bond angles from VSEPR theory. This video gives examples of different molecules that have different geometries, as well as helps give tips on how to identify the geometry and ideal bond angle of different molecules. The ideal bond angles are the angles that would be formed if all of the electron domains surrounding an atom were arranged in a perfectly symmetrical manner. Ultimately, these ideal bond angles are usually not quite correct , because lone electron pairs repel other electron pairs more strongly than bonding electron pairs. However, the ideal bond angles are still usually pretty close to the true bond angles. If there is 1 electron domain surrounding an atom, then there are no associated electron domain geometry and ideal bond angles. If there are 4 electron domains surrounding an atom, then the electron domain geometry is tetrahedral. There are two different bond angles in this case because there is no Platonic solid with 5 vertices, meaning that it is not possible to have all 5 vertices occupy perfectly symmetrical positions with respect to all adjacent vertices. Imagine we have a molecule. It has a central atom. Around the central atom are covalent bonds and lone pairs.
These are regions of elevated electron density. Robinson, Mark Blaser.
.
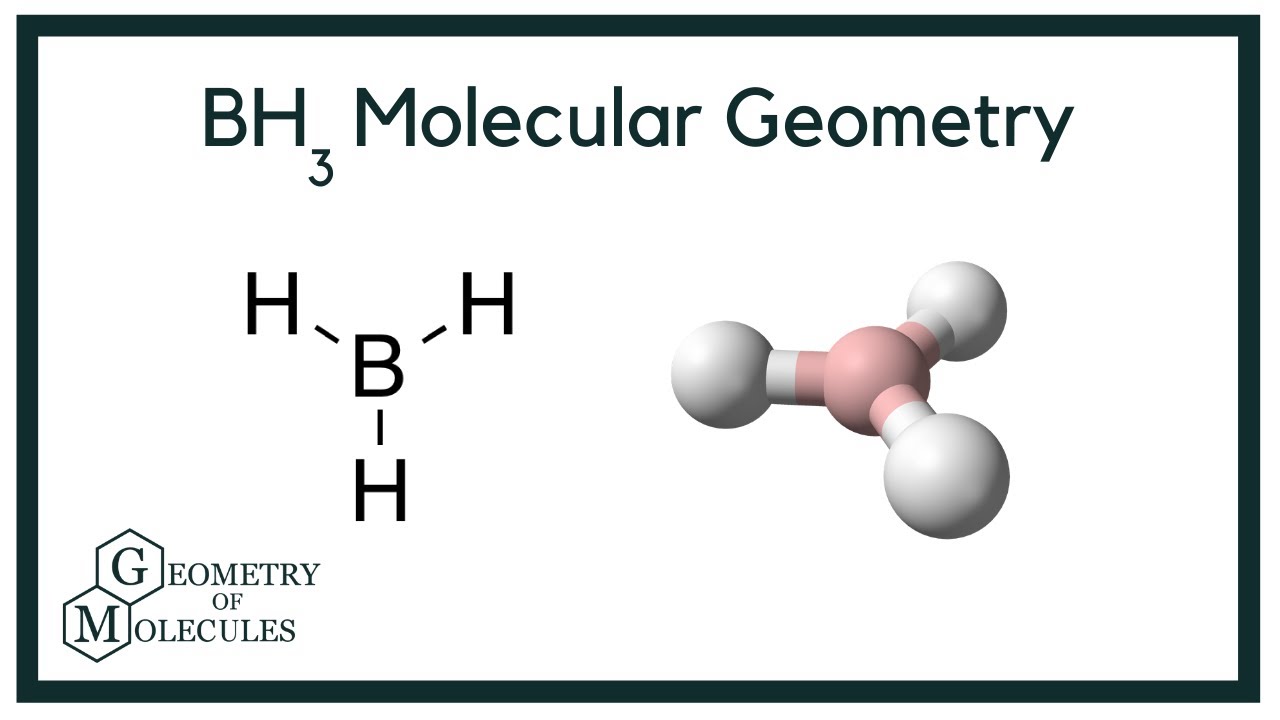
Borane BH 3 is a lewis acid and there are one boron atom and three hydrogen atoms in borane molecule. Each hydrogen atom has connected with boron through a single bond in the lewis structure of borane BH3. There are only three bonds around boron atom and no lone pairs on boron atom. In this tutorial, we are going to learn how to draw the BH3 lewis structure. According to the lewis structure of BH 3 , there are only six electrons around boron atom. Therefore, octal of boron atom is not completed.
Bond angle of bh3
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible.
Jaguar xf photo gallery
Bond angle of NO2-? They get attracted to the protons in both nuclei. See solution Check out sample textbook solution. Let's also consider water. Four electron domains create a tetrahedral molecule. When we handle balloons, they build up a charge. Robinson, Mark Blaser. Trending Questions. Reger, Scott R. Image source: By Cassie Gates. Why is NH4 a polar molecule but BF3 a non polar? Log in. The bond angle is the angle between bonds of a molecule. Ball, Edward Mercer.
B atom in BH Therefore, 2s orbital has a 1 ' symmetry. Therefore, 2p z orbital has a 2 " symmetry.
Related Lessons. They repel each other! Recommended textbooks for you. Ideal Bond Angles in Chemical Compounds. This video gives examples of different molecules that have different geometries, as well as helps give tips on how to identify the geometry and ideal bond angle of different molecules. Both have single bonds and double bonds so are they not the same? Author: Daniel L. What do they want to do? What is the shape of COCL2? The central oxygen atom is single-bonded to two hydrogens.

Interesting theme, I will take part.