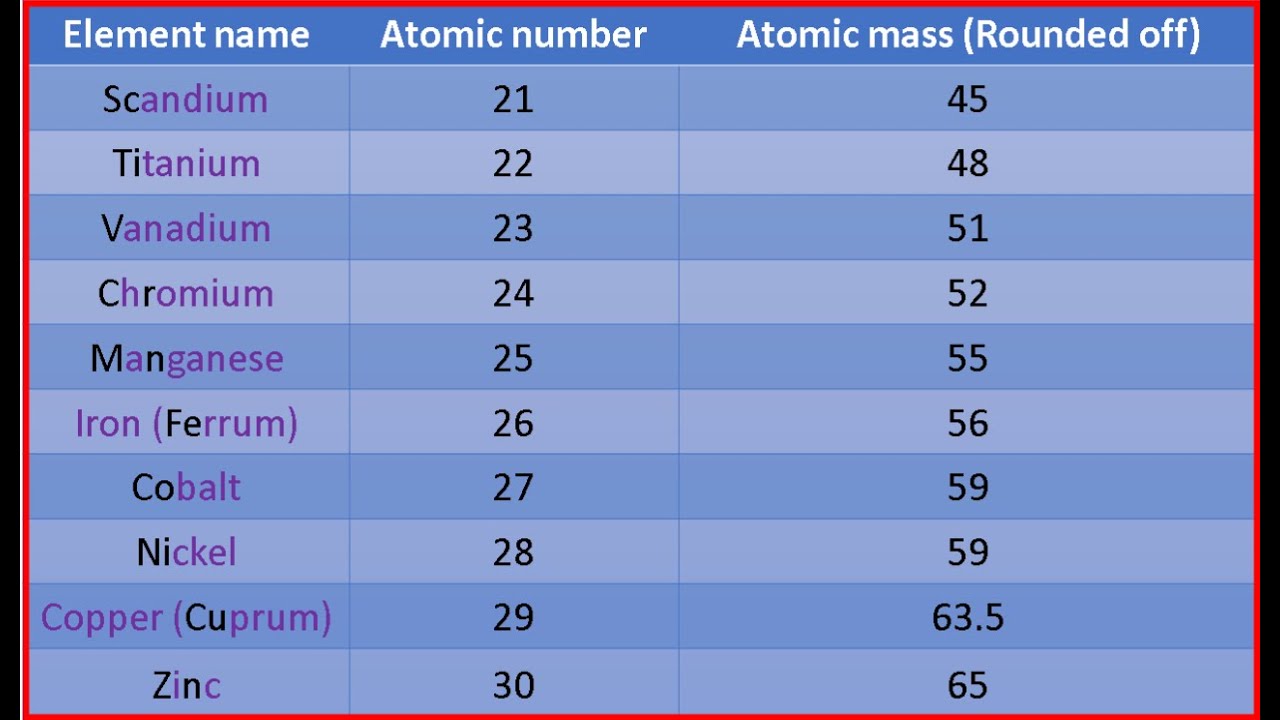
Approx atomic mass of first 30 elements
The atomic mass of elements is measured with the help of unified atomic mass units.
The atomic mass in Chemistry is the average mass of the atoms of an element measured in atomic mass units amu. The atomic mass is simply defined as the weighted average of all of the isotopes of an element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. An interesting point to note is that it is also referred to as atomic weight. In this article, we will learn about the following things: the atomic mass of elements in detail, what is the atomic mass of all elements, and what is the atomic number and atomic mass of elements. Since we have seen the definition of atomic mass let us discuss it in detail.
Approx atomic mass of first 30 elements
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next. What is Scribd? Academic Documents. Professional Documents. Culture Documents. Personal Growth Documents. Uploaded by Prithvi Bhardwaj. AI-enhanced title and description. Document Information click to expand document information This document lists the atomic numbers, element symbols, and atomic masses of the first 30 elements.
Here are three ways to determine the Atomic Mass, depending on the given conditions:. What is Plasma and Bose-Einstein Condensate? What are Divalent Ions?
Atomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. One dalton is equivalent to one-twelfth of the mass of a carbon atom at rest in its ground state. This definition provides a standard reference point for measuring atomic masses. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. This relationship helps simplify calculations and understanding of atomic masses. Atomic Mass of an element is a measure of the average mass of its atoms.
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg. They differ only because a 24 Mg atom has 12 neutrons in its nucleus, a 25 Mg atom has 13 neutrons, and a 26 Mg has 14 neutrons. Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2 H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3 H, is also called tritium and sometimes symbolized T. Use this Build an Atom simulator to build atoms of the first 10 elements, see which isotopes exist, check nuclear stability, and gain experience with isotope symbols. Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number a whole number. However, the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes.
Approx atomic mass of first 30 elements
Atomic mass of all elements along with the rounded off values is mentioned in the chart below. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro. Jay holds the roles of an author and editor at Periodic Table Guide, leveraging his ability to provide clear explanations on typically unexciting topics related to periodic table. He is passionate to help student, and he finds immense joy in his endeavors to make learning enjoyable and accessible. You can connect with him on facebook and twitter. View all posts. Save my name, email, and website in this browser for the next time I comment. The elements whose atomic masses are written in bracket are the synthetic elements and their atomic masses values represent the Atomic Mass of the most stable isotope.
Synonym for abusive
For example, the elements in Group 1A are mostly soft metals that are highly reactive with water. Functional Glass Coatings: George E. What is the Atomic Mass of Neon? Harshita September 26, at pm. This article is being improved by another user right now. Share your suggestions to enhance the article. Difference between Electrovalency and Covalency. Chapter 1 Final Chapter 1 Final. Atomic mass is also used in the classification of different isotopes of the same element Only isotopes of an element share the same atomic number. Atoms of the same element that have a different number of neutrons are called isotopes. Carousel Next. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Cations vs Anions What are Ionic Compounds?
The atomic mass of elements is measured with the help of unified atomic mass units. One unified atomic mass unit can be quantified as the weight of one-twelfth of the mass of a carbon atom considering that it is at rest. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.
Your Mobile number and Email id will not be published. Skip to content. The atomic mass of an individual atom is closely related to its mass number, which represents the total number of protons and neutrons in the nucleus. Atomic mass is the average mass of the protons, neutrons, and electrons in an atom. Effects Of Deforestation. Test your Knowledge on Atomic Mass of Elements! In the same group, a periodic repetition of properties can be seen in the elements with increasing mass. Additional Information. You can suggest the changes for now and it will be under the article's discussion tab. What is Fractional Atomic Mass? Let us now learn about the difference between the atomic number of elements and their atomic mass.

Earlier I thought differently, I thank for the help in this question.
I regret, that I can help nothing. I hope, you will find the correct decision. Do not despair.
I apologise, but, in my opinion, you are not right. Let's discuss it. Write to me in PM.