Aluminium sulfate ionic formula
Al 2 SO 4 3 is a chemical compound with the chemical name Aluminium sulphate. Aluminium sulphate is also called Filter Alum or Dialuminum trisulphate. It is a white crystalline solid in its anhydrous form and in its solution form it appears as a colourless liquid, aluminium sulfate ionic formula.
Aluminium sulfate is a double sulfate salt of aluminium usually available in hydrated form. This is an Inorganic salt that is made from the neutralization reaction of Aluminium Hydroxide and sulfuric acid. It is an edible substance used for drinking water purification, as a pickling agent, and as one of the components in baking powder. Aluminium sulfate is also used as a chemical that enhances the immune response. It reduces the growth of bacteria on the skin. Aluminium sulfate is also used as a preservative. However, Using it in excess may lead to some skin irritations also.
Aluminium sulfate ionic formula
.
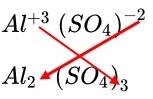
The ionic bond is formed between Aluminium and Oxygens from Sulfate ions. Important Links.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice. Each ion is surrounded by ions of opposite charge. The formula for an ionic compound follows several conventions. First, the cation is written before the anion.
Aluminium sulfate ionic formula
Aluminium sulfate is a salt with the formula Al 2 SO 4 3. It is soluble in water and is mainly used as a coagulating agent promoting particle collision by neutralizing charge in the purification of drinking water [3] [4] and wastewater treatment plants , and also in paper manufacturing. The anhydrous form occurs naturally as a rare mineral millosevichite , found for example in volcanic environments and on burning coal-mining waste dumps.
Minecraft sky texture pack
How dangerous is aluminium sulphate? Due to Reverse Osmosis water, the minerals present in the water are also eliminated along with the impurities. So, purifying the water becomes easy. The solution of aluminium sulphate is corrosive to aluminium. On decomposing, it emits toxic fumes of sulphur oxides. The First Law Of Thermodynamics. Explore SuperCoaching Now. Is Aluminium sulfate acid or alkali? It makes the cement mix correctly without any leaks. For more conceptual understanding, download the Testbook app. Some of them are listed below: As a Pickling agent The agent in the pickling spices that gives the firmness and the crunch is called Alum, also known as aluminium potassium sulfate or potassium aluminium sulfate.
Explore the properties, applications, potential hazards, and environmental implications of aluminum sulfate in this comprehensive guide.
Dinitrogen Trioxide N 2 O 3. Each Aluminium ion is bonded to the two oxides, each of the two sulfate ions like a bridge. As an enhancing agent of the immune system Along with the disease-causing microorganisms, unrelated chemicals called Adjuvants are also added to the vaccines to enhance the response of the Immune system. The solution that results is then evaporated and allowed to crystallize. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Your Mobile number and Email id will not be published. What Is Silicon. Aluminium sulfate is a very good Coagulant which makes it used as a Waterproof agent in construction work. Start Quiz. FREE Signup. Put your understanding of this concept to test by answering a few MCQs. Aluminium sulfate is an inorganic salt formed due to ionic bonds between the metal ions and the sulfate ions. Aluminium sulphate is an irritant to the skin and eyes, so you should wear gloves and eye protection while dealing with it. Antiseptics and anti-bacterial agents help to prevent the growth of microorganisms on the skin. In this section we will discuss the various physical and chemical properties of Aluminium Sulphate.

It is remarkable, it is the amusing information