Acetylene lewis structure
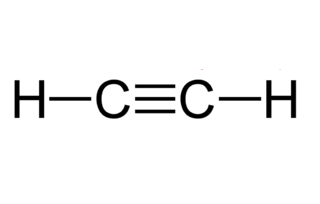
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds, acetylene lewis structure. There are no lone pairs on carbon or hydrogen atoms.
Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colorless, flammable gas with the chemical formula C2H2. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. It also requires the least amount of oxygen to ensure complete combustion. This flammable, colorless gas is lighter than air, so it does not accumulate at low levels, where it could cause a potential hazard.
Acetylene lewis structure
.
This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics.
.
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor. It is highly reactive to atmospheric temperature and lacks oxygen being an unsaturated compound due to the presence of two carbon atoms bonded with a triple bond. As acetylene is reactive, unstable, and lighter than the air, the gas is highly flammable and leads to an explosion. Irrespective of being toxic, acetylene is used for welding purposes as it is flammable. To human beings, this compound is no less than an element of risk as to the existence of it in the atmosphere reduces the level of oxygen. Not only it affects human beings but other living species as well disturbing various natural atmospheric cycles for whom oxygen is an integral component. In light of the same, the recommended airborne exposure limit REL of acetylene is set to ppm Ceiling where an amount greater than this can kill human beings by becoming an asphyxiant gas. With this, it becomes crucial to understand the behavioral chemical properties of acetylene to understand why it behaves in such a specific manner. Lewis Structure is the pictorial representation showing how the valence electrons are participating in bond formation.
Acetylene lewis structure
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1. All of these are sigma bonds. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. This means, in the case of ethane molecule, that the two methyl CH 3 groups can be pictured as two wheels on a hub, each one able to rotate freely with respect to the other. The sp 3 bonding picture is also used to described the bonding in amines, including ammonia, the simplest amine. Just like the carbon atom in methane, the central nitrogen in ammonia is sp 3 -hybridized. With nitrogen, however, there are five rather than four valence electrons to account for, meaning that three of the four hybrid orbitals are half-filled and available for bonding, while the fourth is fully occupied by a non-bonding pair of electrons.
Corrie and co barrow
Now we know how many electrons are includes in valence shells of hydrogen and carbon atomss. What is Phenoxyacetic acid used for? Now, we are going to draw that C 2 H 4 lewis structure step by step. Because C 2 H 2 molecule is a simple molecule, those all steps may not be used. To be the center atom, ability of having greater valance and being a electropositive element are important facts. It is generally supplied dissolved in acetone or DMF. However, you can learn basic examples of drawing lewis structures. An anyone carbon atom can be considered a central carbon. Hybridization of C2H2 The hybridization of carbon atoms in the acetylene C2H2 molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. The Lewis Structure of C2H2 is shown below:. If there are charges on atoms and if those charges can be reduced by converting lone pairs to bonds, we should do that to obtain the best stable lewis structure. The electrons that form bonds are called bonding pairs of electrons, while those that do not take part in any bond formation are called lone pairs or non-bonding pairs of electrons. C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms.
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms.
There are only two sigma bonds around a carbon atom. Carbon is a group IVA element in the periodic table and contains four electrons in their last shell. This flammable, colorless gas is lighter than air, so it does not accumulate at low levels, where it could cause a potential hazard. In the last structure, there are no charges on atoms. It is a colorless, flammable gas with the chemical formula C2H2. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. Now we know how many electrons are includes in valence shells of hydrogen and carbon atomss. Related articles Related Qustion. Also one carbon atom with -2 charge has 2 lone pairs which can be converted to make bonds with other carbon atom to reduce charges. There are only two elements in C 2 H 2 ; hydrogen and carbon. It also requires the least amount of oxygen to ensure complete combustion. In the case of sp hybridization, the s orbital of the central atom only binds with one of its p orbitals. Total electron pairs are determined by dividing the number total valence electrons by two. Those steps are used in detail in this tutorial to draw C 2 H 2 lewis structure. There are several steps to draw a lewis structure of a molecule.

Yes, really. I join told all above. We can communicate on this theme.