Acetic acid lewis dot structure
Weekly Web Work 7: a. Submissions are no longer accepted.
Lewis Dot Structures. This assignment is past due and can no longer be submitted. The purpose of this assignment is to practice drawing Lewis dot structures. You will need to have a pencil and paper to use while working on this assignment. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons. Carbon has 4, oxygen has 6, and hydrogen has 1 valence electron.
Acetic acid lewis dot structure
.
The next step is to write carbon as the central atom. How many valence electrons does carbon dioxide have?
.
It corrodes both metals and tissues. Long-term acetic acid exposure can cause serious irritation in the eyes, skin, nose, throat, and other body parts, among other things. When acetic acid reaches 40 degrees Celsius, it becomes flammable and explosive. In its liquid state, acetic acid is a polar or protic solvent. Lewis structure , also known as electron dot structure, aids in understanding how atoms or valence electrons are grouped in a molecule.
Acetic acid lewis dot structure
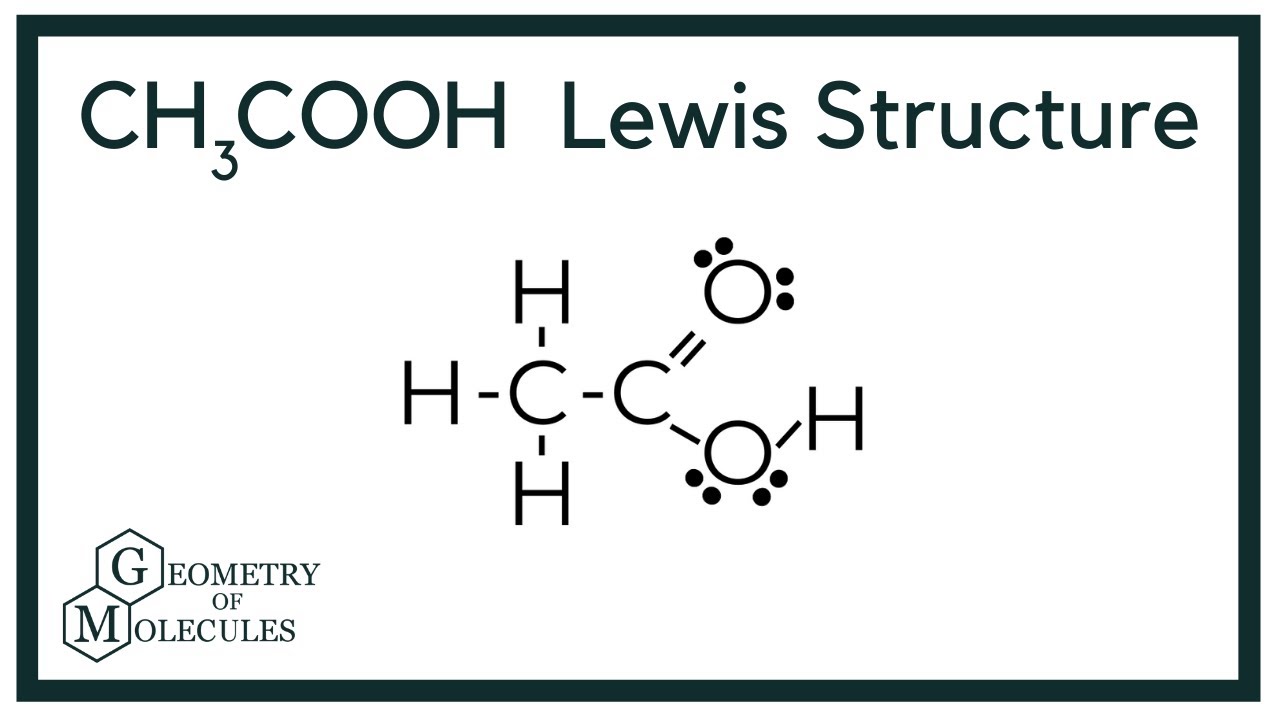
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. But as per the rule, we have to keep hydrogen outside. So, carbon which is less electronegative than oxygen should be placed in the center and the remaining hydrogen atoms as well as COOH group will surround it.
Flats for rent in omr
Weekly Web Work. Chloroform, CHCl 3 , was an early anesthetic. You may change your mind as often as you wish. Examine your initial attempt to draw the Lewis structure of chloroform. Submissions are no longer accepted. Do you see 24 valence electrons? Now, place hydrogen and chlorine atoms around the carbon. Draw a bond between carbon and each surrounding atom. Remember, since this file is in the archive, you can no longer submit it. The purpose of this assignment is to practice drawing Lewis dot structures.
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons.
Do you see 24 valence electrons? So, two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons. There are 6 single bonds and one double bond in this structure and two lone pairs of electrons on each oxygen. Submissions are no longer accepted. Now, place hydrogen and chlorine atoms around the carbon. Does the total number of electrons in your Lewis structure match the number of valence electrons actually available? Does the total number of electrons in your Lewis structure match the number of valence electrons actually available? Now, place hydrogen and chlorine atoms around the carbon. If not, you need to add lone pairs to satisfy each atom. There are 6 single bonds and one double bond in this structure and two lone pairs of electrons on each oxygen. Our Chemical World. If not, you need to add lone pairs to satisfy each atom. Remember, since this file is in the archive, you can no longer submit it. How many valence electrons does chloroform have? In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons.

I consider, that you are mistaken. Write to me in PM.