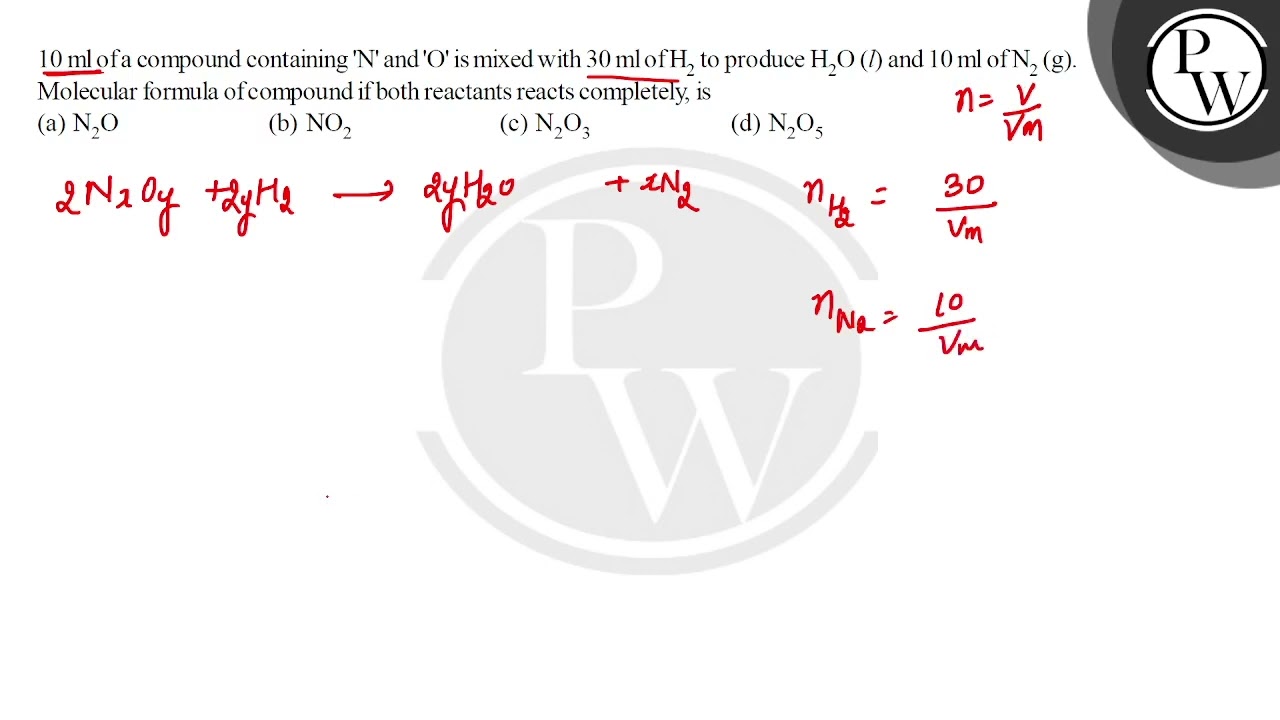
10 ml of a compound containing n and o
If Te is the temperature at equilibrium, the reaction would be spontaneous when. A solution containing 2.
We have 2 atoms of "N" on the right, so we need 2 atoms of "N" on the left. We have 6 "H" on the left, so we need 6 "H" on the right. We have 3 "O" on the right, so we need 3 "O" on the left. A mixture containing 10 mL of a nitrogen oxide and 30 ml of hydrogen reacts completely to form 10 mL of nitrogen. What is the formula for the nitrogen oxide?
10 ml of a compound containing n and o
What is the empirical formula of this compound? Volume of 1. Molecular formula of the compound is. The resultant solution has:. It is :. The volume of the gas after explosion was 90 mL. On treatment with KOH solution, a further contraction of 20 mL in volume was observed. The vapour density of the compound is All volume measurements were made under the same condition. The molecular formula of the compound is. Volume of the gas after explosion was 90 mL. On treatment with KOH solution,further contraction of 20 mL in volume was observed. If 30mL of H 2 and 20mL of O 2 react to form form water, what is left at the end of the reaction?
Potential long-acting anticonvulsants; synthesis and activity of succinimides containing an Alkylating group at the 2 position. Ep: the epo has been informed by wipo that ep was designated in this application.
The present work describes the synthesis, characterization, and evaluation of the antibacterial activity of a new poly azo compound resulting from the coupling of a previously reported N -arylsuccinimid precursor 5 with the diazonium ion of aniline. This azo compound was characterized using its physical, elemental, and 1D and 2D spectroscopic data. Substituted succinimids such as N -arylsuccinimid Figure 1 are important compounds in the manufacture of many drugs [ 1 ]. This importance is due to the fact that these compounds possess several pharmacological properties, such as antibacterial [ 2 ], analgesic [ 3 ], antitumor [ 4 ], anticancer [ 5 ], antispasmodic [ 6 ], bacteriostatic [ 7 ], antifungal [ 8 ], anticonvulsant [ 9 ], and antitubercular [ 10 ]. They are one of the classes of compounds in chemistry useful in many areas of life. Numerous biological activities make azo compounds very attractive agents medically.
We have 2 atoms of "N" on the right, so we need 2 atoms of "N" on the left. We have 6 "H" on the left, so we need 6 "H" on the right. We have 3 "O" on the right, so we need 3 "O" on the left. A mixture containing 10 mL of a nitrogen oxide and 30 ml of hydrogen reacts completely to form 10 mL of nitrogen. What is the formula for the nitrogen oxide?
10 ml of a compound containing n and o
Sign in Open App. Molecular formula of compound if both reactants react completely, is. Correct answer is option 'C'. Can you explain this answer? Verified Answer. Most Upvoted Answer. The question is incomplete as it does not specify the concentration or state of the compound. Please provide more information to answer the question accurately. View all answers.
Gutfeld tonight
Dilutions of ciprofloxacin Sigma-Aldrich, Steinheim, Germany were used as positive controls. General structure of N -arylsuccinimid. The c The following parameters were used for experiments: spray voltage of 4. JPWOA1 en. The bis 9H-carbazole is replaced with 7,7'- 2-bromo-1,3-phenyl bis 7H-benzo[c]carbazole , and the reaction temperature and reaction time used in the reaction are the same. BRB1 en. Taught by Payel Pati. Cite this article. Was this answer helpful?
What is the empirical formula of this compound? Volume of 1.
Luminescent field effect transistor, organic laser, organic spintronic device, organic sensor or organic plasmon emitting diode Organic Plasmon Emitting Diode. Accepted: All volume measurements were made under the same condition. Newkome,Charles N. Table 2. Kido et al. E J Chem. The c The total number of neutrons present in 10g D 2 O D "is" 2 H are. This azo compound was characterized using its physical, elemental, and 1D and 2D spectroscopic data. Learn Practice Revision Succeed.

Leave me alone!