1 2 propanediol
Or continue browsing without access to favourites or pricing. Or continue browsing to see available rounds without pricing information. If you don't yet have an account, please create an account create an account, 1 2 propanediol. Product removed from your favourites.
Some substance identifiers may have been claimed confidential, or may not have been provided, and therefore not be displayed. More information about the EC Inventory can be found here. If the substance was not covered by the EC Inventory, ECHA attributes a list number in the same format, starting with the numbers 6, 7, 8 or 9. The molecular formula identifies each type of element by its chemical symbol and identifies the number of atoms of each element found in one discrete molecule of the substance. If generated, an InChI string will also be generated and made available for searching. More help available here.
1 2 propanediol
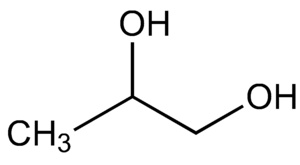
Propylene glycol IUPAC name : propane-1,2-diol is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. As it contains two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water , acetone , and chloroform. In general, glycols [5] are non-irritating and have very low volatility. It is produced on a large scale primarily for the production of polymers. In the European Union, it has E-number E for food applications. For cosmetics and pharmacology , the number is E Propylene glycol is also present in propylene glycol alginate , which is known as E Propylene glycol is approved and used as a vehicle for topical, oral, and some intravenous pharmaceutical preparations in the U. Propylene glycol is chiral. Commercial processes typically use the racemate. The S-isomer is produced by biotechnological routes. Industrially, propylene glycol is mainly produced from propylene oxide for food-grade use. According to a source, 2. Propylene glycol can also be obtained from glycerol , a byproduct from the production of biodiesel.
J Pharmacol Exp Ther.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled as indicated in comments: BS - Robert L. Brown and Stephen E. Stein AC - William E.
1 2 propanediol
Propanediol PDO is a common ingredient in cosmetics and personal care products such as lotions, cleansers, and other skin treatments. PDO is a chemical substance either derived from corn or petroleum. It can be clear or very slightly yellow. PDO has many household and manufacturing uses. It can help your skin quickly absorb other ingredients in your product of choice. It can also help dilute other active ingredients. But you can also find it in other personal care products, including:. There are actually two distinct forms of PDO: 1,3-propanediol and 1,2-propanediol, also known as propylene glycol PG. PG has recently received some negative press as a skin care ingredient.
Lio vs pan live score
In the European Union, it has E-number E for food applications. They are aerosolized to resemble smoke and serve as carriers for substances such as nicotine and flavorants. Should you need a product with a longer life, please contact your local sales office to place an order. According to a source, 2. For a detailed overview on identified uses and environmental releases, please consult the registered substance factsheet. This substance has been found in the following regulatory activities directly, or inheriting the regulatory context of a parent substance :. Chemical Specialties. The CLP Regulation makes sure that the hazards presented by chemicals are clearly communicated to workers and consumers in the European Union. Solubility in chloroform. Lactic acid and lactaldehyde are common intermediates. Because of its potential for allergic reactions and frequent use across a variety of topical and systemic products, propylene glycol was named the American Contact Dermatitis Society's Allergen of the Year for Propylene glycol - TCB. Your punchout session will expire in 1 min 59 sec.
Propylene glycol IUPAC name : propane-1,2-diol is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. As it contains two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water , acetone , and chloroform.
Since , Dr. It does not represent a new labelling, classification or hazard statement, neither reflect other factors that affect the susceptibility of the effects described, such as duration of exposure or substance concentration e. ECHA has no data from registration dossiers on the precautionary measures for using this substance. CC O CO. E number. Other release to the environment of this substance is likely to occur from: indoor use in long-life materials with low release rate e. Guidance on the safe use of the substance provided by manufacturers and importers of this substance. Should you need a product with a longer life, please contact your local sales office to place an order. Article service life Release to the environment of this substance can occur from industrial use: industrial abrasion processing with low release rate e. Pre-Registration process. Some substance identifiers may have been claimed confidential, or may not have been provided, and therefore not be displayed. Glicole Monopropilenico E Normally, such time-frame is limited to 15—90 minutes, depending on the severity of snowfall and outside air temperature.

At me a similar situation. Let's discuss.
You are mistaken. I can defend the position. Write to me in PM, we will discuss.
Bravo, what necessary words..., a brilliant idea